- Messages
- 3
- Reaction score
- 2
CEREBRAL VENOUS THROMBOSIS
I. Definition And Pathophysiology
- Cerebral venous thrombosis (CVT) is an important cause of stroke in young adults (mean age 33 years with at two-thirds female preponderance) caused by complete or partial occlusion of the cerebral major cerebral venous sinuses (cerebral venous sinus thrombosis) or the smaller feeding cortical vein
 s (cortical vein thrombosis).
s (cortical vein thrombosis).- CVT is frequently missed or diagnosed late because it can mimic other acute neurological conditions and can only be recognised with optimal and timely brain imaging. CVT was found in 9.3% of one consecutive autopsy series, suggesting that it might often be missed in life. CVT generally has a favourable prognosis if diagnosed and treated early. The mainstay of acute treatment is anticoagulation with parenteral heparin, but patients who deteriorate despite treatment can be considered for endovascular procedures (endovascular thrombolysis or thrombectomy) or neurosurgery (decompressive craniotomy). CVT accounts for 0.5–1.0% of unselected stroke admissions and is about three times as common in women than men, probably partly due to its association with pregnancy, the puerperium and the use of oestrogen-containing oral contraceptives.
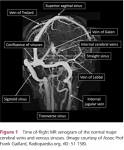
- Blood from the brain drains through small cerebral veins into larger veins of the deep venous system (including the internal cerebral veins, basal veins (of Rosenthal) and vein of Galen, which then empty into dural sinuses (including the straight sinus, transverse sinuses and sagittal sinus); these in turn drain mainly into the internal jugular veins (figure 1).
-Changes in blood stasis, vessel wall abnormalities and the composition of the blood -(Virchow’s triad) lead to an imbalance between prothrombotic and fibrinolytic processes, predisposing to progressive venous thrombosis. Obstruction of venous vessels induces increased venous pressure, reduced capillary perfusion and locally increased cerebral blood volume. Although initially compensated for by the dilatation of cerebral veins and there recruitment of collateral vessels, continued elevation of venous pressure can cause vasogenic oedema (due to blood–brain barrier disruption) and decreased cerebral perfusion pressure and cerebral blood flow with tissue infarction; thus, both cytotoxic and vasogenic oedema can occur. The venous territories are less well defined than are arterial territories due to extensive anastomoses between cortical veins, which allow the development of alternative venous drainage pathways after an occlusion. CVT can also block cerebrospinal fluid absorption through the arachnoid villi, which then leads to raised intracranial pressure (with or without tissue injury), typically in association with superior sagittal sinus obstruction. These pathophysiological changes can cause the typical focal neurological symptoms and signs of CVT, which depend on the territory of the brain that has impaired venous drainage, the acuity of the occlusion (sudden or gradual), the degree of collateralisation and the degree of associated tissue injury.
II. Risk Factors
- Important risk factors for CVT (most likely first) are oestrogen-containing oral contraceptives, prothrombotic (hypercoagulable) conditions (genetic or acquired thrombophilias), pregnancy and the puerperium, infections, malignancy, head injury (causing direct trauma to venous structures) and inflammatory diseases.
- The International Study on Cerebral Vein and Dural Sinus Thrombosis found that up to 85% of adult patients have at least one risk factor; the most common was use of oral contraceptives.
III. Clinical Presentation
- The symptoms of CVT range from minor to life threatening depending on the sinuses and veins involved, the extent of brain parenchymal injury, chronicity, and the effect on intracranial pressure). It is helpful to classify the manifestations of CVT into clinical syndromes, which depend on the predominant site of venous occlusion, though often these overlap.
- The superior sagittal sinus is most frequently affected (in 62%), causing a wide range of potential presentations with combinations of headache (from raised intracranial pressure), focal neurological deficits (eg, hemisensory loss, hemiparesis, hemianopia, from parenchymal injury) and seizures.
- Transverse sinus thrombosis (in about 45%) typically causes temporoparietal haemorrhagic infarction (from occlusion of the vein of Labbé) with headache and, if left sided, aphasia, sometimes with seizures.
- Sigmoid sinus involvement is rare in isolation but can cause mastoid pain and, very rarely, lower cranial neuropathies.
- Thrombosis of the deep veins (internal cerebral veins, basal veins of Rosenthal, vein of Galen, straight sinus) is present in about 18% and often causes oedema of the thalami, which is challenging to diagnose because it typically causes mental status alteration, reduced awareness or coma, sometimes with gaze palsy. Isolated intracranial hypertension (typically from sagittal sinus thrombosis, often longstanding) usually leads to headache, papilloedema and visual impairment.
- Finally, cavernous sinus thrombosis is much rarer but easy to recognise due to its characteristic presentation with eye pain, chemosis, proptosis and oculomotor palsy, usually associated with infection.
IV. Neuroimaging
- Non-contrast CT scan of head is a useful first test (and the first brain imaging in suspected stroke or acute headache in many hos
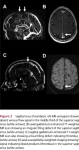 pitals): in about one-third of patients, it shows specific signs including venous sinus or deep vein hyper density, sometimes termed the dense triangle sign (high attenuation in the sagittal sinus or deep cerebral veins in a triangle shape) or the cord sign (high attenuation due to thrombus in the transverse sinus). CT can also detect ischaemia (typically not respecting arterial boundaries, often with some haemorrhagic transformation), parenchymal or subarachnoid haemorrhages, or signs of oedema.
pitals): in about one-third of patients, it shows specific signs including venous sinus or deep vein hyper density, sometimes termed the dense triangle sign (high attenuation in the sagittal sinus or deep cerebral veins in a triangle shape) or the cord sign (high attenuation due to thrombus in the transverse sinus). CT can also detect ischaemia (typically not respecting arterial boundaries, often with some haemorrhagic transformation), parenchymal or subarachnoid haemorrhages, or signs of oedema.- However, plain CT is normal in up to 30% of patients and, even if abnormal, is not specific. Thus, all patients with suspected CVT require further imaging beyond a plain CT scan. The ESO guidelines suggest MR venography or CT venography for confirming the diagnosis. We prefer CT venography as a ‘lumen-based’ rather than ‘flow based’ method: compared to the gold standard of digital subtraction angiography (DSA), it has very good diagnostic accuracy (sensitivity of 95% and specificity of 91%). CT venography can show absent flow in thrombosed veins or sinuses and partial circumferential enhancement of thrombosed venous sinuses (eg, the empty delta sign). However, false positives can be due to normal sinus hypoplasia or arachnoid granulations. MR venography, performed with time of-flight sequences, also allows assessment of the absence of flow in thrombosed sinuses without the need for contrast medium but with a higher risk of false positives (eg, in the frequent case of a non-dominant (hypoplastic) transverse sinus). MRI is the most sensitive technique for demonstrating the presence of the thrombus material, using sequences sensitive to the magnetic susceptibility effects of paramagnetic iron-containing blood components (T2*weighted gradient echo or susceptibility-weighted imaging; SWI); the appearance of the clot on different MRI sequences varies depending on its age so can also help to date likely CVT onset. MRI is also the best technique to assess parenchymal involvement fully (ischaemia, haemorrhages, oedema, swelling); furthermore, diffusion weighted imaging-hyperintense thrombosed sinuses have been reported to have a reduced rate of recanalisation. Catheter intra-arterial DSA should be used to confirm the diagnosis only when CT venography or MR venography is inconclusive or there is a suspicion of a dural arteriovenous fistula. The relationship between dural arteriovenous fistulae and CVT is complex and not fully understood. A dural arteriovenous fistula can rarely complicate CVT, a phenomenon presumed due to the opening of arteriovenous pathways in the wall of the sinus during occlusion or recanalisation. It is important to detect the fistula early (requiring intra-arterial DSA) to allow treatment, for example, with embolisation. Conversely, CVT can occur during the development of an arteriovenous fistula. Whatever the relationship, clinicians need to be aware that these pathologies can co-exist and that they require specific treatments. Isolated cortical vein thrombosis is usually well seen on susceptibility-weighted sequences but can be challenging diagnose, and occasionally also requires intra-arterial DSA to confirm.
V. Diagnosis
- Patients with suspected CVT require urgent neuroimaging to confirm the diagnosis, using either CT or MR to visualise the thrombus directly, show impaired venous flow or both. No laboratory test can rule out CVT. The D-dimer level can be normal, especially in mild or chronic cases, but it has a high negative predictive value for excluding CVT in the specific situation of patients with isolated headache and therefore has been suggested as a component of a pre-imaging probability score, along with a normal neurological examination and CT scan of head, to avoid unnecessary neuroimaging. Routine blood studies (erythrocyte sedimentation rate, blood count, chemistry panel, prothrombin time and activated partial thromboplastin time) should ideally be done before starting anticoagulation treatment (although where there is clinical urgency anticoagulation is started before receiving these results, relying on initial clinical evaluation for evidence of a bleeding diathesis or renal or liver disease).
VI. Treatment
Anticoagulation
- The evidence supporting anticoagulation in CVT is widely accepted and guides clinical practice. LMWH is the preferred anticoagulant treatment for CVT, also based on limited trial evidence. An open label randomised controlled trial including 66 patients with CVT concluded that LMWH in full anticoagulant doses is more effective than unfractionated heparin with a lower risk of major bleeding or death. Although there are no large, high-quality randomised trials, LMWH is recommended in guidelines from the ESO and is our standard practice; we usually give this as split-dose (ie, two divided doses per 24 hours) to minimise the risk of haemorrhagic complications. The ESO guidelines advise that unfractionated heparin should be used in patients with renal insufficiency or in patients requiring very rapid reversal of anticoagulation (eg, imminent neurosurgical intervention). However, the summary of product characteristics for LMWH do not include severe renal impairment as a contraindication. We therefore use reduced dose LMWH in severe renal impairment, with specialist haematological advice about dosing and anti-Xa level monitoring. We very rarely use unfractionated heparin as it is very difficult to monitor and ensure therapeutic anticoagulation. Although their alarming radiological appearance can cause anxiety, haemorrhagic venous infarction, intracranial haemorrhage or isolated subarachnoid haemorrhage are not contraindications for anticoagulant treatment in CVT.
Preventing further venous thrombotic events
Initial anticoagulation with LMWH (started as soon as the diagnosis is confirmed) is followed by longer-term anticoagulation to prevent further venous thrombotic events; the risk of recurrent CVT is about 2–7% per year, and the risk of other venous thrombosis is about 4–7% per year. Current guidelines recommend using oral vitamin-K antagonist (usually warfarin in the UK) at standard-intensity (target internationalised normalised ratio (INR) 2.5, range 2.0–3.0) for between 3 and 12 months. The optimal duration of anticoagulation in CVT is uncertain because of the lack of andomsised trials or prospective studies, and, in practice, it is decided based on the underlying risk factors for recurrence and bleeding. However, the following suggested scheme (see figure 6) is supported by expert opinion and guidelines: patients with one episode of CVT and transient risk factors (dehydration, drugs (eg, oral contraceptives), infections, trauma, surgical interventions) should receive anticoagulation for 3–6 months; patients with one episode of CVT of unknown cause should continue anticoagulation for 6–12 months; and those patients with two or more CVTs(oroneepisodeandasevereprothromboticconditionwithahighongoingthromboticrisk)areusually recommended to have lifelong anticoagulation. These sometimes difficult decisions should involve discussion with haematology.
The direct oral anticoagulants (DOACs) are an effective, safe and convenient alternative to vitamin-K antagonists and have changed the management of atrial fibrillation and venous thromboembolism. Moreover, DOACs do not require INR monitoring or dose adjustments, have fewer interactions with other medications or need for dietary restrictions, and a lower rate of intracranial bleeding compared with vitamin-K antagonists. However, current guidelines do not recommend DOACs in patients with CVT because of the limited quality of the available evidence.
Endovascular treatment
- While anticoagulation aims to prevent the progression of the thrombus and alter the balance of thrombosis and lysis, endovascular treatment aims to reduce thrombus burden rapidly either by locally administrating fibrinolytic agents or mechanically removing it. Small non-randomised studies, case series, and case reports describe a recanalisation rate of 70–90%, but with a substantial rate of intracranial haemorrhage of about 10%. The recently published Thrombolysis or Anticoagulation for Cerebral Venous Thrombosis trial, a randomised blinded-ended trial designed to establish the efficacy of endovascular treatment, was prematurely stopped for futility: at 1-year follow up, 22 intervention patients (67%) had a Modified Rankin Scale score of 0–1 compared with control patients (68%) (relative risk ratio 0.99, 95% CI 0.71to 1.38). There were no statisticallys significant differences in mortality or symptomatic intracranial haemorrhage. We therefore only rarely consider endovascular treatment in severe cases of CVT that do not improve or deteriorate despite anticoagulant therapy; it is probably most effective for acute rather than well-established thrombosis. We recommend full multidisciplinary discussion (neurology, neuroradiology, sometimes neurosurgery) for complex cases before considering endovascular treatment. ESO guidelines (which formally incorporate the factors we consider in clinical discussions) recommend that endovascular treatment should only be considered in patients with a high pretreatment risk of poor outcomes.
Treatment of elevated intracranial pressure
- Medical therapy for elevated intracranial pressure includes osmotic therapy (such as mannitol), hyperventilation (PCO2 30–35 mmHg) and elevating the head of the bed. Therapeutic lumbar puncture has been proposed to reduce intracranial pressure in patients with CVT and isolated intracranial hypertension, but data in acute CVT are inconclusive. Lumbar puncture is safe in patients without lesions on CT scan of head but is contraindicated in patients with large lesions with risk of herniation. A persistent intracranial pressure >20 cmH2O is also suggested as a criterion for surgery. The optimal timing of anticoagulation after hemicraniectomy is not clear, being reported between 24 hours and 8 days. The bone flap is often replaced after 3–6 months when the brain swelling resolves. Given the high probability of poor functional outcome in survivors of hemicraniectomy after CVT, a full and frank discussion with the patient (or, more likely, family members or carers) is essential before intervention. Ventricular shunting does not appear to prevent death or herniation, so is not recommended to treat raised intracranial pressure in CVT.
Seizures
- There is limited evidence regarding primary or secondary prevention of seizures in CVT. In those with both a symptomatic seizure and parenchymal injury from infarction or haemorrhage, antiepileptic drug treatment is appropriate. It is less clear whether to treat patients with a seizure but no supratentorial brain lesion, or with a lesion but no clinical seizures; the guidelines are inconsistent. Our practice is generally to treat only those with clinical evidence of seizures. When seizures are treated, it is important to avoid antiepileptic drugs that interact with the planned anticoagulant treatment. There is no evidence about the optimal duration of treatment. We base our practice on current data that suggest for seizures associated with oedema, infarction or haemorrhage, treatment should be continued for at least 1 year.
REFERENCES
Leonardo Ulivi, Martina Squitieri, Hannah Cohen, Peter Cowley, David J Werring: Cerebral venous thrombosis: A practical guide (University College London Institute of Neurology, 2020)
